Everything we see around us, from the air we breathe to the stars shining in the sky, is made of matter. Matter is defined as anything that has mass and occupies space. While most of us are familiar with solids, liquids, and gases, science tells us that matter exists in five distinct states, each with unique properties governed by energy, temperature, and particle behavior.
- 1. Solid: Matter with Fixed Shape and Volume
- 2. Liquid: Fixed Volume but No Fixed Shape
- 3. Gas: No Fixed Shape or Volume
- 4. Plasma: The Fourth State of Matter
- 5. Bose–Einstein Condensate (BEC): Matter at Near Absolute Zero
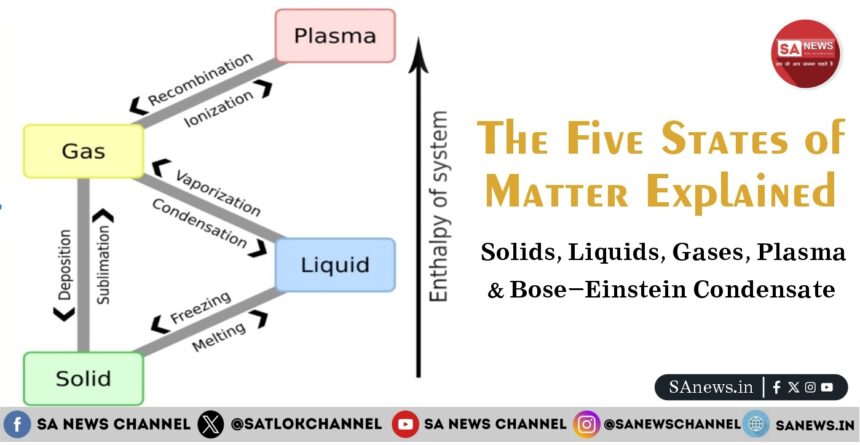
- How Matter Changes from One State to Another
- The Five States of Matter: Insights into the Physical and Cosmic World
- Spiritual Perspective: Beyond Physical Matter
- FAQs: The Five States of Matter (including Plasma)
Understanding these states helps us explain everyday experiences, advanced technologies, and even cosmic phenomena. Let us explore each state of matter in depth.
1. Solid: Matter with Fixed Shape and Volume
Solids are the most familiar state of matter. In a solid, particles (atoms or molecules) are tightly packed and arranged in a fixed structure. Because of this close arrangement, solids have a definite shape and definite volume.
Key Characteristics of Solids
- Strong intermolecular forces hold particles in place
- Particles vibrate but do not move freely
- High density compared to liquids and gases
- Almost incompressible
For example, a stone, a metal rod, or an ice cube retains its shape no matter where it is placed. Even when you apply force, solids usually resist deformation.
Types of Solids
Solids can be classified into:
- Crystalline solids, such as salt and diamonds, where particles are arranged in an orderly pattern
- Amorphous solids, such as glass and plastic, where particles lack long-range order
2. Liquid: Fixed Volume but No Fixed Shape
Liquids occupy a middle position between solids and gases. In liquids, particles are still close together, but they are not fixed in place. This allows liquids to flow and take the shape of their container while maintaining a fixed volume.
Key Characteristics of Liquids
- Definite volume but variable shape
- Moderate intermolecular forces
- Particles slide past one another
- Low compressibility
- Water is the most common example of a liquid. When poured into a glass, it changes shape, but the amount of water remains the same.
Unique Properties of Liquids
- Liquids show special behaviors such as:
- Surface tension, which allows insects to walk on water
- Viscosity, which measures resistance to flow (for example, honey flows more slowly than water)
3. Gas: No Fixed Shape or Volume
Gases are vastly different from solids and liquids. In gases, particles are far apart and move freely at high speeds. Because of this, gases have no definite shape or volume and expand to fill any container.
Key Characteristics of Gases
- Very weak intermolecular forces
- Highly compressible
- Low density
- Rapid and random particle motion
Air is a mixture of gases, mainly nitrogen and oxygen. When you inflate a balloon, gas particles spread evenly inside, pushing against the walls.
Behavior of Gases
- Gas behavior is explained using gas laws such as:
- Boyle’s Law (pressure and volume relationship)
- Charles’s Law (volume and temperature relationship)
A simple explanation of gas laws can be found at Khan Academy: https://www.khanacademy.org/science/chemistry/gases-and-kinetic-molecular-theory
4. Plasma: The Fourth State of Matter
Plasma is often overlooked, yet it is the most abundant state of matter in the universe. Plasma forms when a gas is heated to extremely high temperatures, causing electrons to break free from atoms. This creates a mixture of charged particles.
Key Characteristics of Plasma
- Composed of ions and free electrons
- Conducts electricity
- Responds strongly to magnetic fields
- Emits light
- Unlike ordinary gases, plasma behaves in complex ways because of its electric charge.
Examples of Plasma
- The Sun and stars
- Lightning
- Neon signs and fluorescent lamps
- Auroras near Earth’s poles
Plasma is essential in modern technology, including plasma TVs, medical sterilization, and experimental nuclear fusion reactors.
5. Bose–Einstein Condensate (BEC): Matter at Near Absolute Zero
The fifth state of matter, Bose–Einstein Condensate, exists under extreme conditions. It forms when atoms are cooled to temperatures close to absolute zero (−273.15°C). At this point, atoms lose individual identity and behave as a single quantum entity.
Key Characteristics of Bose–Einstein Condensate
- Extremely low temperature
- Quantum effects visible on a macroscopic scale
- Atoms move in perfect synchronization
- Very low energy state
BEC was first predicted by Albert Einstein and Satyendra Nath Bose in the 1920s and was experimentally achieved in 1995.
Why is BEC Important?
- Studying BEC helps scientists:
- Understand quantum mechanics
- Explore superconductivity
- Develop advanced sensing technologies
How Matter Changes from One State to Another
Matter can change its state through physical processes without altering its chemical identity.
Common Phase Changes
- Melting: Solid to liquid
- Freezing: Liquid to solid
- Evaporation: Liquid to gas
- Condensation: Gas to liquid
- Ionization: Gas to plasma
These changes occur due to the absorption or release of energy, usually in the form of heat.
Why Understanding States of Matter Matters
Knowledge of states of matter is not limited to textbooks. It plays a vital role in:
- Weather prediction and climate science
- Space exploration
- Medical technologies
- Material science and engineering
- From designing spacecraft shields to understanding blood flow in the human body, the principles of matter guide innovation.
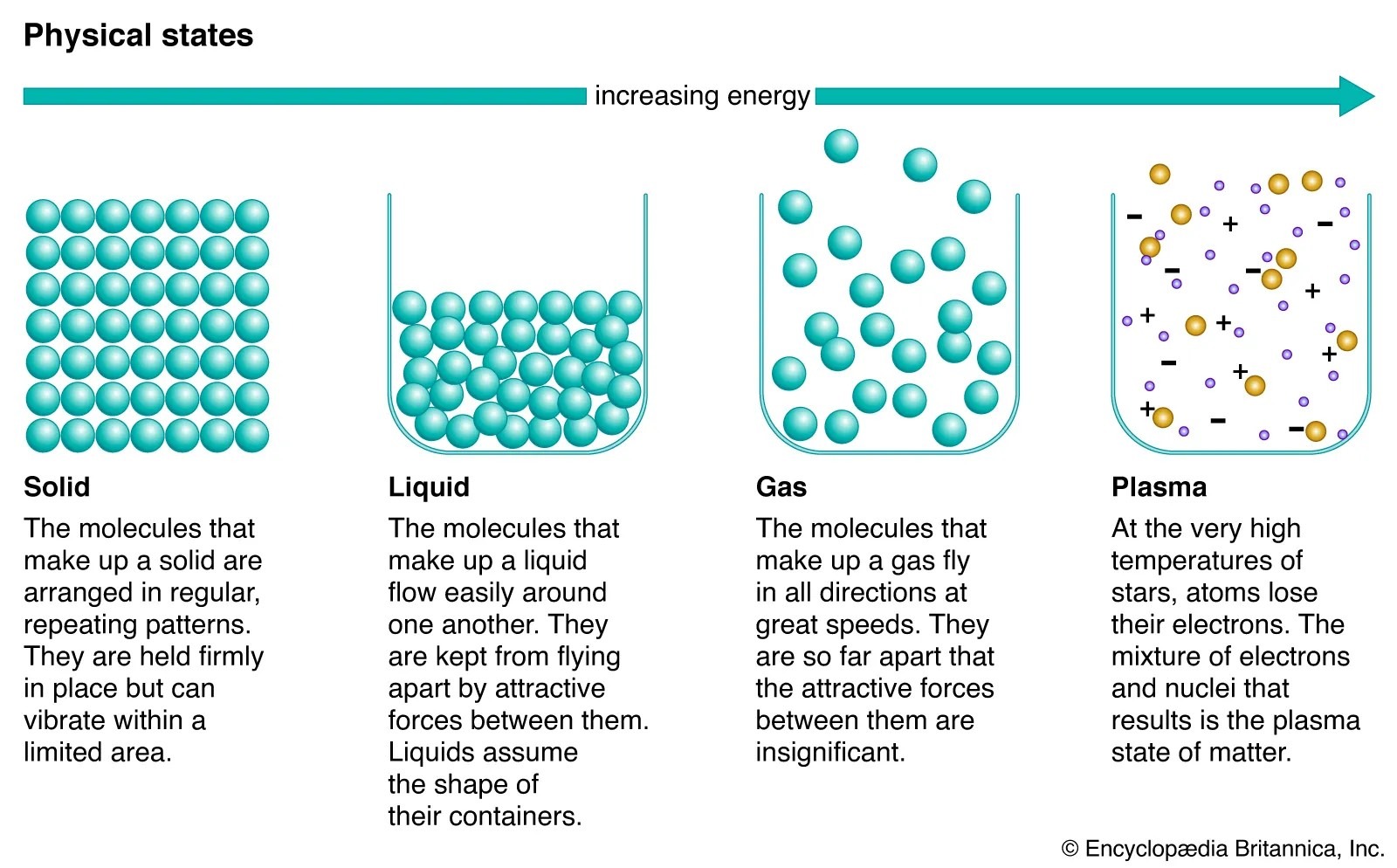
Image Source: science photo library
The Five States of Matter: Insights into the Physical and Cosmic World
The five states of matter; solid, liquid, gas, plasma, and Bose–Einstein condensate—represent different ways particles organize and behave under varying conditions of energy and temperature. While solids, liquids, and gases shape our daily experiences, plasma dominates the universe, and Bose–Einstein condensates open doors to quantum discoveries.
By understanding these states, we gain deeper insight into both the visible world around us and the invisible forces that govern the cosmos.
Spiritual Perspective: Beyond Physical Matter
While science explains the five states of matter through temperature, energy, and particle behavior, the spiritual knowledge given by Jagatguru Tatvdarshi Sant Rampal Ji Maharaj Ji takes our understanding far deeper. According to true spiritual wisdom, this entire material universe including solid, liquid, gas, plasma, and even subtle quantum states is temporary and destructible.
Holy scriptures clarify that all physical matter exists within the realm of Kaal Brahm, where creation and destruction are continuous. Even advanced states like plasma or Bose–Einstein condensate remain part of this perishable system. This is why scientific progress, though valuable, cannot grant permanent peace or liberation to the soul.
Sant Rampal Ji Maharaj Ji explains that the soul is not made of material elements. It is eternal and originates from Satlok, the everlasting world of the Supreme God Kabir. True liberation is not achieved by mastering material science, but by understanding the correct method of worship revealed by a Tatvdarshi Saint, as described in sacred scriptures.
Thus, while science helps us understand how matter behaves, spiritual knowledge answers why existence itself was created and how the soul can escape this temporary material world and attain eternal salvation.
For readers seeking deeper insight into the creation of nature and the universe, it is highly recommended to read the “Creation of Nature” by Jagatguru Rampal Ji Maharaj ji. This would provide detailed spiritual knowledge about the origin, formation, and purpose of the material world according to the teachings of Sant Rampal Ji Maharaj Ji.
FAQs: The Five States of Matter (including Plasma)
1. What makes plasma different from a regular gas?
Plasma differs from gas because it consists of charged particles (ions and electrons), which allow it to conduct electricity and respond to magnetic fields. While gases have neutral particles, plasma emits light and is the dominant state of matter in the universe, found in stars and lightning.
2. Can all substances exist in all five states of matter?
Not all substances are observed in every state. Solids, liquids, and gases are common for most materials, but plasma requires extremely high energy and BEC occurs only near absolute zero. Only certain experimental conditions can produce these extreme states.
3. Why is Bose–Einstein Condensate called the fifth state of matter?
BEC is considered the fifth state because it exhibits unique quantum properties that are not seen in solids, liquids, gases, or plasma. At near absolute zero, atoms act collectively as one coherent quantum entity, showing behaviors that defy classical physics.
4. How do phase changes affect the properties of matter?
Phase changes, such as melting, freezing, evaporation, condensation, or ionization, alter the arrangement and movement of particles without changing the substance chemically. These changes explain why water can exist as ice, liquid water, or vapor under different temperatures.
5. How does spiritual wisdom view the states of matter?
According to Jagatguru Tatvdarshi Sant Rampal Ji Maharaj Ji, all physical states, including plasma and BEC, are temporary and perishable. Spiritual knowledge reveals that the soul is eternal, and understanding the true purpose of creation, as explained in the book Gyan Ganga, is far more important than mastering matter alone.