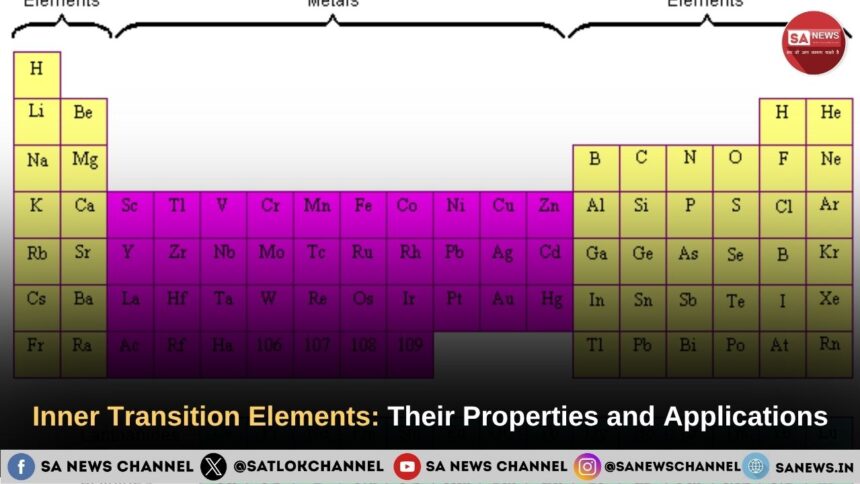
Inner transition elements are elements in which electrons are filled in the inner f-subshell of the atom. They are divided into two series: lanthanides and actinides. One of their main properties is that they form colored compounds. They are arranged in two series, exhibit magnetic properties, and are used as catalysts.
Properties of inner transition elements:
Electronic configuration: In these elements, the last electron enters the f-subshell, giving rise to a variety of chemical reactivity and other properties.
Colored compounds: Compounds of many inner transition elements are colored because their f-electrons have specific energy levels.
Magnetic properties: Many inner transition elements exhibit magnetic properties because their f-electrons attract them to a magnetic field.
Catalytic activity: Many inner transition elements are used as catalysts to speed up chemical reactions.
High melting points: These elements generally have high melting and boiling points.
Metallic properties: They are generally shiny, hard, and good conductors of heat and electricity.
What is an internal transition element?
Inner transition elements are elements in which the last electron enters the f-shell. They are usually found in group 3 of the periodic table but are listed separately as f block elements.These f block elements are known as inner transition elements.
There are two series of inner transition elements:
- Lanthanide series – When the last electron enters the 4f orbital.
- Actinide series – When the last electron enters the 5f orbital.
In this article, we will study the main characteristics of the inner transition elements. Also, the differences and similarities between lanthanides and actinides.
Applications of Inner Transition Elements:
- Nuclear weapons: Elements like uranium and plutonium are used to make nuclear explosives. Naturally occurring isotopes of uranium are unstable, making them highly reactive.
- Nuclear power plants: They are used in the construction and operation of nuclear reactors.
- Lasers: Lanthanides are used in laser production.
- Geological dating: Elements like samarium and lutetium are used to determine the age of fossils and rocks.
- Magnets: Lanthanides are used to manufacture strong permanent magnets.
- Sunglasses: They are used in glass coloring and UV protection in sunglasses.
- Medicinal uses: Certain elements are used to destroy targeted cancer cells.
- Uranium is used as a radiation shield.
- It is also used as a tracer in diagnostic processes.
FAQs
What is the transition principle?
Ans: Transition elements are those whose atoms or ions have partially filled d-orbitals.
What are the principles of f block?
Ans: The f-block consists of the lanthanide (4f) and actinide (5f) series of elements.
What is the chemical symbol of sodium?
Ans: Na
What are transition metals?
Ans: Iron (Fe), Copper (Cu), Zinc (Zn), Silver (Ag), and Gold (Au)